Recognising the importance of chronic lung disease: a consensus statement from the Global Alliance for Chronic Diseases (Lung Diseases group)


“Peer-reviewed report on Moderna COVID-19 vaccine publishes - National Institutes of Health” plus 4 more |
| Peer-reviewed report on Moderna COVID-19 vaccine publishes - National Institutes of Health Posted: 30 Dec 2020 01:38 PM PST 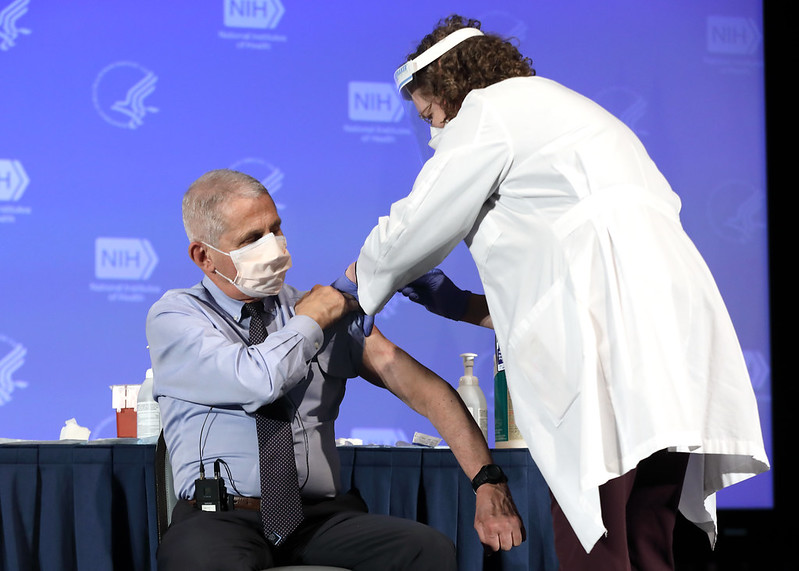 News Release Wednesday, December 30, 2020 Data from Phase 3 clinical trial confirm vaccine is effective. WhatThe investigational vaccine known as mRNA-1273 was 94.1% efficacious in preventing symptomatic coronavirus disease 2019 (COVID-19), according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine. The vaccine also demonstrated efficacy in preventing severe COVID-19. Investigators identified no safety concerns and no evidence of vaccine-associated enhanced respiratory disease (VAERD). The vaccine was co-developed by Moderna, Inc., a biotechnology company based in Cambridge, Massachusetts, and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Moderna and NIAID previously shared initial results from the COVE trial. On Dec. 18, 2020, the FDA issued an Emergency Use Authorization allowing Moderna to make the vaccine available for the prevention of COVID-19 in adults in the United States. The trial was led by principal investigators Lindsey R. Baden, M.D. of Brigham and Women's Hospital in Boston, Hana M. El-Sahly, M.D. of Baylor College of Medicine in Houston, and Brandon Essink, M.D., of Meridian Clinical Research. The trial was implemented under the U.S. government's Operation Warp Speed program and supported by NIAID and the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services' Office of the Assistant Secretary for Preparedness and Response. The trial began on July 27, 2020, and enrolled 30,420 adult volunteers at clinical research sites across the United States. Volunteers were randomly assigned 1:1 to receive either two 100 microgram (mcg) doses of the investigational vaccine or two shots of saline placebo 28 days apart. The average age of volunteers is 51 years. Approximately 47% are female, 25% are 65 years or older and 17% are under the age of 65 with medical conditions placing them at higher risk for severe COVID-19. Approximately 79% of participants are white, 10% are Black or African American, 5% are Asian, 0.8% are American Indian or Alaska Native, 0.2% are Native Hawaiian or Other Pacific Islander, 2% are multiracial, and 21% (of any race) are Hispanic or Latino. From the start of the trial through Nov. 25, 2020, investigators recorded 196 cases of symptomatic COVID-19 occurring among participants at least 14 days after they received their second shot. One hundred and eighty-five cases (30 of which were classified as severe COVID-19) occurred in the placebo group and 11 cases (0 of which were classified as severe COVID-19) occurred in the group receiving mRNA-1273. The incidence of symptomatic COVID-19 was 94.1% lower in those participants who received mRNA-1273 as compared to those receiving placebo. Investigators observed 236 cases of symptomatic COVID-19 among participants at least 14 days after they received their first shot, with 225 cases in the placebo group and 11 cases in the group receiving mRNA-1273. The vaccine efficacy was 95.2% for this secondary analysis. There were no concerning safety issues with vaccination, according to the authors. Local reactions to the vaccine were generally mild. About 50% of participants receiving mRNA-1273 experienced moderate-to-severe side effects — such as fatigue, muscle aches, joint pain and headache — after the second dose, which resolved in most volunteers within two days. Investigators also observed no evidence of VAERD among those who received mRNA-1273. This rare complication was seen in individuals vaccinated with a whole-inactivated respiratory syncytial virus (RSV) vaccine in the 1960s, before there was a capacity to define protein structures and measure immune responses with precision. VAERD can occur when a vaccine induces an immune response that is not strong enough to protect against infection. Although mRNA-1273 is highly efficacious in preventing symptomatic COVID-19, there is not yet enough available data to draw conclusions as to whether the vaccine can impact SARS-CoV-2 transmission. Preliminary trial data suggests there may be some degree of prevention of asymptomatic infection after a single dose. Additional analyses are underway of the incidence of asymptomatic infection and viral shedding post-infection to understand the vaccine's impact on infectiousness. The authors concluded by discussing the unprecedented efficiency of the candidate vaccine's development, noting, "this process demonstrates what is possible in the context of motivated collaboration among key sectors of society, including academia, government, industry, regulators and the larger community." ArticleLR Baden, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England Journal of Medicine. DOI: 10.1056/NEJMoa2035389. WhoNIAID Director Anthony S. Fauci, M.D. is available to comment on this study. John R. Mascola, M.D., director of NIAID's Vaccine Research Center, is also available to comment. ContactTo schedule interviews, please contact the NIAID News & Science Writing Branch, (301) 402-1663, niaidnews@niaid.nih.gov. NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website. About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov. NIH…Turning Discovery Into Health® ### |
| Everything to know about allergic reactions to COVID vaccines - Fortune Posted: 31 Dec 2020 09:10 AM PST  © 2020 Fortune Media IP Limited. All Rights Reserved. Use of this site constitutes acceptance of our Terms of Use and Privacy Policy | CA Notice at Collection and Privacy Notice | Do Not Sell My Personal Information | Ad Choices |
| Posted: 31 Dec 2020 05:30 AM PST  TipRanks 3 Big Dividend Stocks Yielding Over 7%; Raymond James Says 'Buy'Wall Street's investment firms are burning the midnight oil as we approach the end of 2020, publishing their year-end notes and their New Year prognostications, both for investors' edification. There is the obvious point: we're in a moment of rising markets, and investor sentiment is riding high now that the election is settled and COVID vaccines have emergency approval and are getting into the distribution networks.However, the lockdown policies put in place to combat the virus this winter are slowing down the economic recovery. Whether the economy will truly tank or not is yet to be seen.In the meantime, Raymond James strategist Tavis McCourt has published his take on the current situation, and his comments bear consideration. First, McCourt notes the investors are focused on the good news: "[The] equity market is more focused on vaccine deployment and complete re-openings of economies in 2021, and so far, negative data points have been largely brushed aside."Looking ahead, McCourt writes of the next two years: "We believe the logical outcome of 2021 (and 2022 for that matter) is a likely "return to normalcy" with strong EPS growth offset by lower P/Es barring a change in the vaccine story. We expect cyclical sectors and smaller cap equities to continue to outperform, as is typical in early cycle markets…"The research analysts at Raymond James have been searching the markets for the 'right' buys, and their picks bear a closer look. They've been tapping high-yielding dividend payers as an investment play of choice.The TipRanks database sheds some additional light on three of JMP's picks – stocks with dividends yielding 7% or better – and that the investment firm sees with 10% upside or better.New Residential Investment (NRZ)The real estate investment trust (REIT) segment has long been known for its high and reliable dividends, a feature promoted by tax regulations which stipulate that these companies must return a certain proportion of profits directly to investors. Based in New York City, New Residential Investment is typical of its sector. The company's portfolio includes residential mortgages, mortgage loan servicing rights, and loan origination. NRZ focuses its operations on the residential housing sector.NRZ is a mid-cap company, with a market value of $4.13 billion and a portfolio worth $5.72 billion. The company's revenues have been rising since the second quarter of 2020, after steep losses during the 'corona recession' of Q1. The third quarter earnings, however, came in at 19 cents per share, down from 54 cents in the year-ago quarter. But even with that loss, NRZ took care to maintain the dividend.In fact, it did more than that. The company raised the Q3 dividend, to 15 cents per common share, in a continuation of an interesting story. Back in Q1, the company pared back the common share dividend to 5 cents, in a move to preserve capital during the corona crisis. The company has since raised the dividend by 5 cents in each subsequent quarter, and the Q4 payment, announced in mid-December, is for 20 cents per common share. At that rate, the dividend annualizes to 80 cents and the yield exceeds 7.87%.In addition to raising the dividend, NRZ has also announced a share buyback program totaling $100 million. The repurchase is for preferred stock shares, and goes alongside the existing repurchase policy of common shares.Analyst Stephen Laws, in his coverage of NRZ for Raymond James, writes, "We expect strong origination volumes and attractive gain on sale margins to drive strong near-term results, and we continue to expect a dividend increase in 4Q [...] For 4Q20, we are increasing our core earnings estimate by $0.02 per share to $0.35 per share. For 2021, we are increasing our core earnings estimate by $0.08 per share to $1.31 per share."In line with these comments, Laws rates the stock an Outperform (i.e. Buy). His $11.50 target price implies a one-year upside of 16%. (To watch Laws' track record, click here)It's not often that the analysts all agree on a stock, so when it does happen, take note. NRZ's Strong Buy consensus rating is based on a unanimous 8 Buys. The stock's $11.36 average price target suggests a 14% and a change from the current share price of $9.93. (See NRZ stock analysis on TipRanks)Fidus Investment Corporation (FDUS)Next up is a business development corporation, Fidus Investment. This company is one of many in the mid-market business financing niche, offering debt solutions and capital access to smaller firms that may not be able to secure lending from the larger markets. Fidus' portfolio focuses on senior secured debt and mezzanine debt for companies valued between $10 million and $150 million.Fidus has investments in 68 companies with an aggregate value of $697 million. The largest portion of that portfolio, 59%, is second-lien debt, with the rest divided mainly between subordinated debt, first-lien debt, and equity-related securities.The company has seen revenues gain through the second and third quarters of 2020, after negative results in Q1. The third quarter top line came in at ~$21 million, up an impressive 129% sequentially. Since the third quarter, Fidus has declared its dividend for Q4, at 30 cents per common share, the same as the previous two quarter, plus an extra 4-cent special dividend authorized by the Board of Directors. This brings the total payment for the quarter to 34 cents per common share, and puts the yield at 9.5%.Raymond James analyst Robert Dodd likes what he sees in Fidus, especially the dividend prospects. "We continue to see the risk / reward as attractive at current levels - with shares trading below book, solid forecasted base dividend coverage from NII… We project FDUS solidly over-earning its quarterly base dividend of $0.30 / share through our projection period. As a result, we do project modest supplementals…"Dodd puts an Outperform (i.e. Buy) rating on the stock, and sets a target price of $14. At current levels, that target indicates an upside of 10.5% in the next months. (To watch Dodd's track record, click here)Wall Street is somewhat more divided on FDUS shares, a circumstance reflected in the Moderate Buy analyst consensus rating. That rating is based on 4 reviews, including 2 Buys and 2 Holds. Shares are priced at $12.66, and the $13.33 average price target suggests a modest 5% upside from current levels. (See FDUS stock analysis on TipRanks)TPG RE Finance Trust (TRTX)Returning to the REIT sector, we look at TPG RE Finance Trust, the real estate financing arm of global asset firm TPG. This REIT, with an $820 million market cap, has built a portfolio of commercial mortgage loans worth an aggregate total of $5.5 billion. The company is a provider for original commercial mortgage loans starting at $50 million, mainly in US primary markets. The largest share of the company's loans and properties are centered in the East.Like many finance companies, TPG RE Finance saw serious losses in Q1 due to the corona pandemic crisis – but has since recovered to a large extent. Revenues in Q3 hit $48 million, up 9% year-over-year. During the quarter, TPG received loan repayments totaling $199.6 million, a solid result, and when the quarter ended the company had on hand $225.6 million in cash or cash equivalents.The company was able to easily fund its dividend, of 20 cents per common share, in Q3. For Q4, the company has recently declared not just the 20-cent regular payment, but also an 18-cent non-recurring special cash dividend. Taken together, the dividends give a yield of 7.5%, almost 4x higher than the average found among S&P-listed companies.Returning to Raymond James' REIT expert Stephen Laws, we find that he is bullish on TRTX, too. "TRTX has underperformed since reporting 3Q results, which we believe creates an attractive buying opportunity… We expect core earnings to continue benefiting from LIBOR floors in loans and expect new investments to resume in 1Q21. The company's portfolio has combined retail and hotel exposure of 14%, which is below the sector average of 19%..." To this end, Laws rates TRTX a Strong Buy and his $13 price target suggests ~22% upside in 2021. (To watch Laws' track record, click here)This stock also holds a Strong Buy rating from the analyst consensus, based on 3 unanimous Buy reviews set in recent weeks. Shares are priced at $10.67 and the average target of $11.00 suggests a modest 3% upside from current levels. (See TRTX stock analysis on TipRanks)To find good ideas for dividend stocks trading at attractive valuations, visit TipRanks' Best Stocks to Buy, a newly launched tool that unites all of TipRanks' equity insights.Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment. |
| Posted: 31 Dec 2020 05:15 AM PST LITTLE RIVER, S.C.--(BUSINESS WIRE)--PCT Ltd. (OTC Pink:PCTL) announces that PCTL and RB Capital Partners, Inc. have executed and received an additional $150,000 conventional, convertible loan with 5% annual interest and a $0.10/share fixed conversion rate. PCTL has earmarked a large portion of these monies for the build and completion of 4 Annihilyzer® Infection Control Systems for the Healthcare Industry and the completion of 3 pieces of the Company's Legacy equipment. With regard to the Company's issued and outstanding shares; as of today, our I/O share count has remained the same at 721,187,846 shares. As PCTL continues its pursuit to gain acceptance to list on OTC:QB, we are in the final stages of addressing all comments from the OTC Markets Compliance Team. Provided there are no more questions, the process will proceed in queue with OTC Markets for the jump to the OTC:QB listing. Finally, as seen on our Tweet yesterday, we will additionally be disseminating pertinent company information on our official Twitter account, (https://mobile.twitter.com/@PCTL2021). Later today, the Company will Tweet information about our end-of-year shareholder letter and will provide a link to our website for review of that communication. About PCT LTD: PCT LTD ("PCTL") focuses its business on acquiring, developing and providing sustainable, environmentally safe disinfecting, cleaning and tracking technologies. The company acquires and holds rights to innovative products and technologies, which are commercialized through its wholly-owned operating subsidiary, Paradigm Convergence Technologies Corporation (PCT Corp). Currently trading on OTC:PINK, "PCTL" is actively engaged in applying for listing its common stock to the OTC QB market. The Company established entry into its target markets with commercially viable products in the United States and now continues to gain market share in the U.S. and U.K. ADDITIONAL NEWS AND CORPORATE UPDATES: PCTL would like to warn its stockholders and potential investors that material corporate information regarding sales, areas of business and other corporate updates will only be made through press releases or filings with the SEC and through Twitter (PCTL@PCTL2021). PCTL does not utilize social media, chatrooms or other online sources to disclose material information. The public should only rely on official press releases, Tweets from the Company's official Twitter account, and corporate filings for accurate and up to date information regarding PCTL. Forward-Looking Statements: This press release contains "forward-looking statements" as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21B of the Securities Exchange Act of 1934, as amended. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact and may be "forward-looking statements." Such statements are based on expectations, estimates and projections at the time the statements are made that involve a number of risks and uncertainties, which could cause actual results or events to differ materially from those presently anticipated. Such statements involve risks and uncertainties, including but not limited to: PCTL's ability to raise sufficient funds to satisfy its working capital requirements; the ability of PCTL to execute its business plan; the anticipated results of business contracts with regard to revenue; and any other effects resulting from the information disclosed above; risks and effects of legal and administrative proceedings and government regulation; future financial and operational results; competition; general economic conditions; and the ability to manage and continue growth. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual outcomes may vary materially from those indicated. Important factors that could cause actual results to differ materially from the forward-looking statements PCTL makes in this press release include market conditions and those set forth in reports or documents it files from time to time with the SEC. PCTL undertakes no obligation to revise or update such statements to reflect current events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. |
| Posted: 31 Dec 2020 04:39 AM PST  A new multiplex polymerase chain reaction (PCR) panel was able to match the performance of quantitative bacterial culture and provide greater specificity, according to a new study. The findings raise the possibility for significantly faster, and more accurate, diagnosis of lower respiratory tract infections (LRTIs). The report was published in the Journal of Clinical Microbiology. Corresponding author Matthew D. Sims, MD, PhD, of Beaumont Hospital, in Michigan, and colleagues, wrote that quantitative bacterial culture of bronchoalveolar lavage (BAL) fluids is a lengthy process, and its sensitivity is relatively low, particularly when the patient has had prior antimicrobial therapy. As a result, many patients are empirically prescribed broad-spectrum antibiotics, a problem that contributes to antibiotic resistance. The investigators said an improvement in testing could lead to a significant improvement in patient care. "Early diagnosis and proper choice of antimicrobials are crucial for successful management of pneumonia," they wrote. The new panel, called the Unyvero LRT BAL leverages molecular diagnostics and bioinformatics to detect 19 bacteria, 10 antibiotic resistance markers, and the fungus Pneumocystis jirovecii in BAL samples in roughly 4.5 hours. An earlier study found the panel performed well compared to quantitative bacterial culture in a study of 175 specimens. This new study was designed to verify those results, using 1400 patient samples from 11 clinical trial sites. The majority of the samples (1016) were collected prospectively within 24 hours of arrival at 9 US hospitals. Another 392 archived specimens were also included in the study. The samples were collected between 2015 and 2018 at 2 additional US hospitals. The investigators found an overall positive percent agreement (PPA) between the Unyvero panel and BAL culture of 93.4%, and a negative percent agreement (NPA) of 98.3. For P. jirovecii, the investigators found a 100% (5/5) PPA and a 99% (99/100) NPA. That's a particularly meaningful result, they wrote, because P. jirovecii pneumonia is often not considered as a potential diagnosis; they said the Unyvero panel is the only US Food and Drug Administration (FDA) approved panel to offer such testing, and therefore might allow for more comprehensive diagnosis of this particular type of pneumonia. The study also was better able to detect numerous other clinically relevant pathogens in a rapid manner, which Sims and colleagues said could make it easier for physicians to choose the appropriate therapies and antibiotics, allowing them to better align with antibiotic stewardship goals. In a press release, Faranak Atrzadeh, MA, chief marketing and scientific affairs officer of the panel's developer, OpGen Inc., said microbiological confirmation of severe pneumonia is "a crucial step" for tailoring antibiotic therapy. "Unyvero LRT panels are culture-independent and provide the speed and sensitivity that may achieve more reliable identification of causative agents than culture, allowing clinicians to make more informed decisions about antibiotics use," she said. In terms of antibiotic resistance, the authors compared the panel's antibiotic resistance marker results to standard antibiotic susceptibility testing and found positive predictive values of 80-100%, depending on the particular microorganism and specific resistance marker. The FDA approved the Unyvero LRT panel for use with BAL samples back in January. |
| You are subscribed to email updates from "infection inc" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment